Greyledge is a service company that develops biologic products in the Regenerative Medicine space.
We process material (blood or bone marrow) from humans in a highly controlled, FDA registered laboratory environment, into implantable preparations for medical use. We collaborate with doctors and scientists to create a biologic processing lab on-site to further develop and apply these products into injured or diseased tissue in an effort to stimulate healing and repair above and beyond what is normally possible.
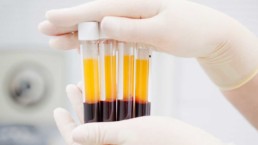
Our primary end-products are PRP (platelet rich plasma) and BMC (bone marrow concentrate).
Both are under very active development and study for Orthopedic applications, with published research to date, suggesting encouraging potential for use and additional refinement.
Platelet Rich Plasma
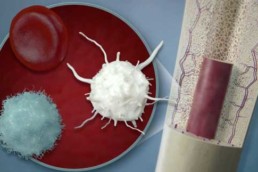
PRP concentrates a patient’s platelets and certain blood cells, which release concentrated proteins after implantation, that coordinate and stimulate the healing process in our bodies.
Bone Marrow Concentrate
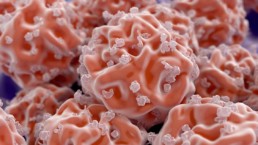
BMC is a similar blood concentrate, but also contains populations of stem cells which can become other cell types and also release proteins to communicate directly with other cells to promote healing and repair.
Greyledge was a company built from an idea that we could treat Orthopedic problems better than we did historically by blocking or inhibiting the body’s response to injury. Greyledge preparations are produced from a patient’s own tissue and are put back into the same patient in an effort to produce a healing effect.
By measuring and studying the exact compositions of PRP and BMC, Greyledge sets up a process of identifying, standardizing and adapting these preparations to become more effective for a patient and their specific problem (like arthritis or a sports injury).
Greyledge was piloted and developed for 6 years in Vail, Colorado undergoing constant refinement and is now expanding strategically with physicians, scientists and health care systems across the country in an effort to improve and refine the practice of Regenerative Medicine. Greyledge protocols are FDA registered and audited. We are engaging to create an electronic quality management system to link our network of clinical partners and allow us to ensure quality from all of our lab operations. The intent with our growth strategy is to create a network of users implanting quality controlled biologics from which we can compare patient outcomes to learn, optimize and adapt by observing trends for success and failure and targeting more specific research from what is observed.
What makes Greyledge unique is our mission of creating detailed quality control programs to measure exactly how many cells and cell types are within our preparations, so we can gain understanding of how to optimize the composition of blood and marrow products to stimulate healing reactions.